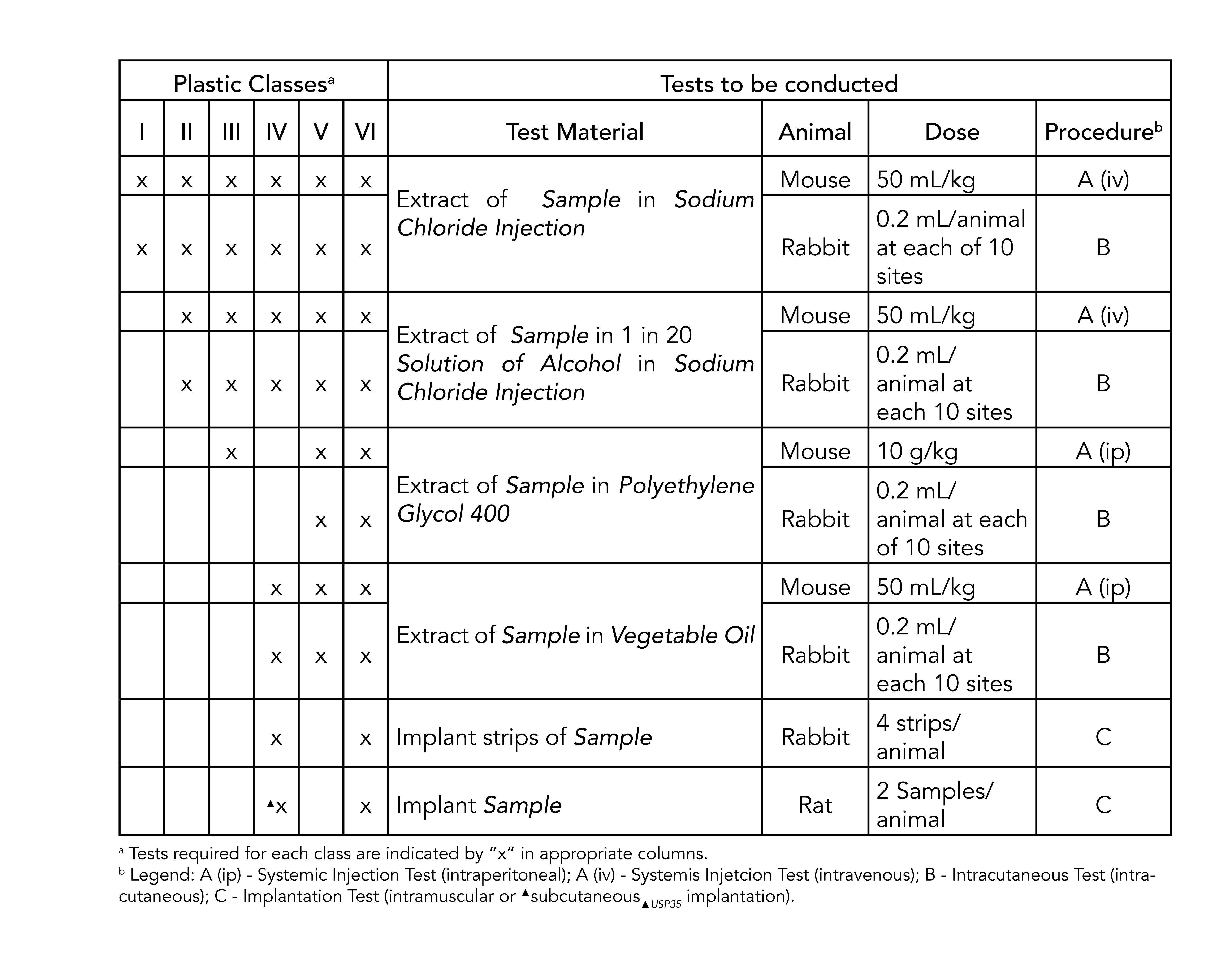
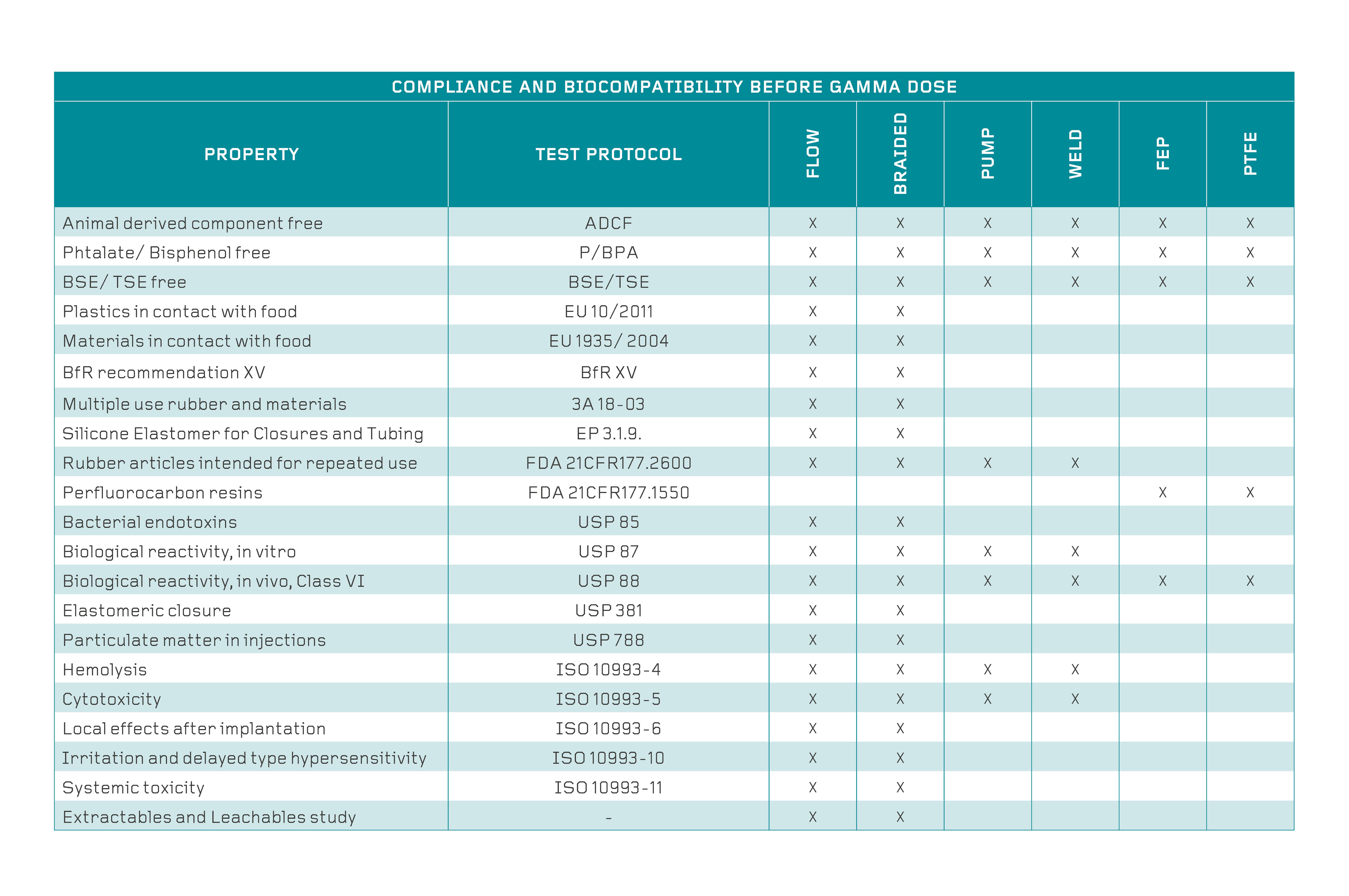
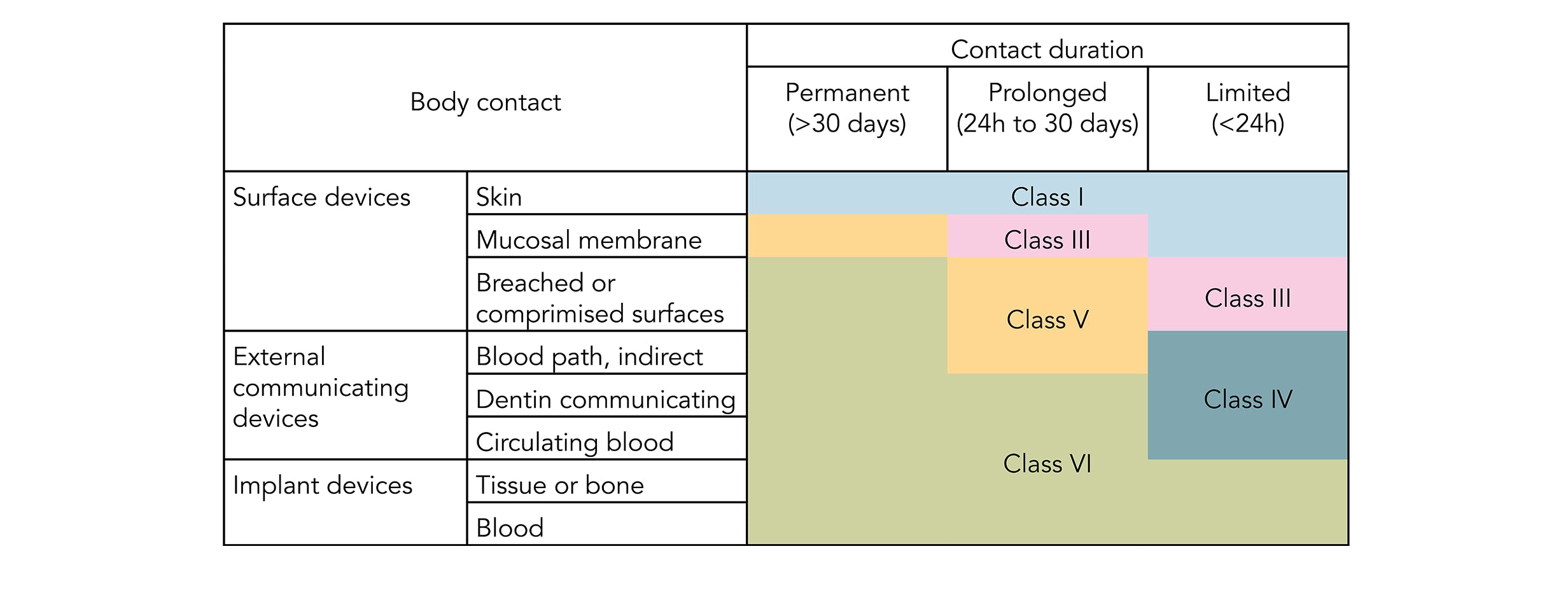
usp class vi elastomers
Sealable and weldable either pre- or post-sterilization C-Flex 072 provides prolonged pump life Sterilizable by gamma irradiation and autoclave Product Validation Test Summaries available upon request Moldable bondable and formable for single-use assemblies and overmolds Temperature. FDA CFR 175300.
038283959 infoeriksbe wwweriksbe ERIKS sa Avenue JE Lenoir 2a 1348 Louvain-La-Neuve tél.
. UL 94V-0. USP Class VI refers to a set of biocompatibility testing requirements from the US. For most patient-contact applications a material that meets US Pharmacopeia USP Class VI andor ISO 109933 will be required.
Ecdel elastomers are plasticizer free copolyester elastomers COPE that offer clarity toughness flexibility and chemical resistance needed in a variety of flexible packaging including flexible medical and pharmaceutical packaging applications. Most applications are fairly benign to elastomers. Pharmacopeia USP and the Food and Drug Administration FDA.
UL 1203 for explosion-proof. It is used in the assembly of medical devices and is capable of withstanding repeated sterilizations including radiation ethylene oxide chemical sterilants and especially autoclaving. ISO 10993-5 for cytotoxicity.
These materials may be blended if desired to achieve intermediate hardnesses. The resulting elastomers range in hardness from soft to firm nominally 35 to 65 shore A durometer. What are Class VI elastomers.
At the end of the test period the cells are examined for cytopathic. Discusses identification testing A workshop Modernization of USP Packaging Standards for Glass and. Inner Dimension - 0375.
Meets USP Class VI and FDA Requirements The elastomer that NAMSA tested SSP-2390 is a platinum-cured silicone that meets requirements from both the US. Explains basic functional characteristics of components 4. As defined in the US.
Usp Class Vi What Is It And How Does It Apply To Elastomers Barnwell Fda And Usp. Specialty Silicone Products SSP makes USP Class VI silicones for medical applications. The Premium Package includes all elastomers in the wetted path of the SLA Series Biotech including O-rings and Valve Seats are USP Class VI and ADI Free and include a full package of certificates.
Something that is listed as being USP Class VI demonstrates that the materials utilized are biologically compatible when tested according to the US. Designates baseline requirements 5. For this reason the FDA provides a standard 21 CFR1772600 defining allowable rubber compound ingredients and extractibles based on toxicity and carcinogenicity.
Ecdel 9966 elastomer meets ISO 10993 andor USP Class VI biocompatibility requirement Ecdel elastomers are plasticizer free copolyester elastomers COPE that offer clarity toughness flexibility and chemical resistance needed in a variety of flexible packaging including flexible medical and pharmaceutical packaging applications. USP Class VI for biocompatibility. The first stage is described as USP Biological Reactivity Tests in vitro.
Additionally 22 Material Certifications certifying the composition of all materials in the wetted path and International Calibration Traceability calibrations traceable to NIST or. The transparent elastomer is a high-purity compound with low leachables and extractables as required by the USP Class VI standard for ensuring patient safety. 010483589 infoeriksbe wwweriksbe ERIKS sas.
To keep up with the changes medical device designers and manufacturers need elastomers they can trust. Pharmacopoeia Class VI judges the suitability of plastic material intended for use as containers or accessories for parenteral preparations. Moulded O-rings class 1 less than 10 furnace black These can be produced in all possible dimensions up to diameter 1400 mm internal.
Tubing USP Class VI Thermoplastic Elastomer meets requirementsfor plastic containers and closures Female MPC and Polycarbonate meets USP Class VI requirementsfor plastics. Has a full range of specialty adhesives epoxies primers for polyolefins UV curables and silicones that have been fully tested to meet USP Class VI requirements. Provides a high-level introduction to elastomer chemistry manufacturing technology and the post processing of components 3.
Master Bond systems are very versatile and can be used for both disposable and reusable medical devices. FDA and USP class VI compliant O-rings High purity elastomers for pharmaceutical biochemical and food industries ERIKS nv Boombekelaan 3 B-2660 Hoboken tel. MIL-STD-883J for thermal stability.
FAR 25853a for flame retardancy. Table 1 shows our standard programme FDA compliant com- FDA and USP class VI compliant. The SSP2390 family of products isnt new but the marketplace for these materials continues to evolve.
Sil 714001 USP class VI Silicone 1 70 Yes transl. Elasto Proxy custom-fabricates silicone seals for medical devices and equipment. All these special grade products have passed this rigorous test.
Liveo Class VI Elastomers C6- eries are a series of one-part uncatalyzed silicone elastomer raw materials. USP Class VI ISO 10993-5 Cytotoxicity In-Vitro Features Benefi ts. Sil 714002 USP class VI Silicone 1 70 Yes transl.
UV10TKMed is USP Class VI approved and meets ISO10993-5 for cytotoxicity requirements. Essentially an extract of the sample or a surface of the sample itself is placed in contact with a healthy monolayer of cultured L929 mammalian fibroblasts for several days. Pawling Engineered Products Inc.
SSP-2390 is safe for gamma sterilization and wont leach extractables at elevated temperatures. Pharmacopoeia XXII materials which pass the Class VI Plastic Evaluation are suitable as implantable materials. Ecdel 9966 elastomer meets ISO 10993 andor USP Class VI biocompatibility requirement.
MIL-STD-810G for fungus resistance. Both ISO 10993 and USP Class VI define testing requirements for biocompatibility the ability of a material to perform a desired function without causing adverse effects on the human body. Medical device makers and medical equipment manufacturers need silicone seals that are made from FDA USP Class VI.
The high-purity elastomers that we use are platinum-cured and FDA USP Class VI and RoHS compliant. Pharmacopoeia XXII 1190 Class VI Plastics Evaluation. PlasticsToday Staff Sep 17 2015 The flexibility durability neutrality and recyclability of the MT-1205I TPE make it a desirable alternative to PVC and thermoset rubber for medical tubing according to Polymax.
What are Class VI elastomers. Engineered Rubber Products Engineering Medical.

Fda And Usp Class Vi O Rings Guide 2020 Nes

Usp Class Vi O Rings And Seals Eastern Seals Uk Ltd

Parker Medical Fda O Rings Sealing Devices

Parker V1274 75 Usp Class Vi Biocompatibility O Ring United Seal
![]()
Ssp 2390 Series Specialty Silicone Products Inc

Fda Usda Nsf51 Usp Class Vi Compliant Seals Products

Meaning Of Usp Class Vi Standard United Kingdom

Usp Class Vi What Is It And How Does It Apply To Elastomers Barnwell

O Rings Fda And Usp Class Vi Darcoid Rubber Company Oakland California
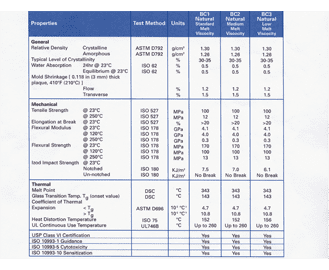
Meaning Of Usp Class Vi Standard United Kingdom

Usp Class Vi What Is It And How Does It Apply To Elastomers Barnwell
Polymaxtpe Receives Usp Class Vi Approval For New Thermoplastic Elastomer In Medical Applications

Why You Need Certified Usp Class Vi Silicones Specialty Silicone Products Inc

Iso 10993 Vs Usp Class Vi Medical Molding And Bicompatible Rubber The Rubber Group